Clinical Need and Commercial Opportunity
Prostate cancer is the most common cancer in men but is very heterogeneous. Few patients die from prostate cancer, however, current tests do not provide sufficient granularity to distinguish indolent (slow growing) from locally advanced to very aggressive cancer. For clinicians and patients, this presents a significant challenge in shared decision making for the best clinical actionability and results in significant overtreatment and overdiagnosis of prostate cancer. Genomic and proteomic approaches have not addressed this challenge, underscoring the great need for a test to augment Gleason testing.
For instance, 70 percent of men diagnosed with low or intermediate grade prostate cancer elect definitive treatment (surgery or radiation) rather than active surveillance (also known as watchful waiting) even though many of these patients’ cancer will remain indolent. The uncertainty created by the imprecision of Gleason scores in assessing which tumors are likely to progress underlies these decisions. The Gleason pathological grading system is based on formalin-fixed paraffin embedded (FFPE) prostate biopsy tissue samples that are microscopically evaluated by a board-certified pathologist.
Novel Phenotypic Test
Cellanyx and clinical collaborators have published (hover popup: URL to Urology 2018, Albala et.al.) the first Cellanyx and clinical collaborators have published (hover popup: URL to Urology 2018, Albala et.al.) the first studies demonstrating the potential of a novel, live cancer cell phenotypic test to predict adverse pathology and allow risk stratification of patients with solid tumors such as prostate and breast cancer. This first-in-class, live tumor cell phenotypic test is designed to provide actionable information on cancer aggressiveness to support shared clinical decision-making. A study, published in Nature Biomedical Engineering, reports initial results from the analytical validation of this novel phenotypic test from a multicenter, blinded clinical study of prostate tissue samples from patients who had undergone radical prostatectomy. The authors additionally describe preliminary results from a separate proof-of-principle study in breast cancer patients undergoing surgery. Predictive metrics from the live tumor cell phenotypic analysis were compared with the conventional post-surgical adverse pathology findings to establish the test’s sensitivity and specificity. Results showed that the Cellanyx generated superior results in predicting adverse pathologies with sensitivity and specificity of more than 85% (ROC >85%) and separated patients into distinct, quantifiable groups based on predicted adverse pathology features.
Cellanyx’s LDT is designed to address the clinical challenges in prostate cancer risk-stratification and treatment plan decisions. This is particularly important for risk stratification in men with low and intermediate grade prostate cancer (Gleason 6 or 7) and/or for patients that have a negative, prostate pathological score at biopsy, while having an elevated prostate surface antigen (PSA) score.
The costs of Gleason score’s imprecision as a stand-alone test, to patients and the healthcare system, are enormous. Tens of thousands of men undergo treatment annually for cancers that do not present a significant risk and can be managed by active surveillance. These patients are exposed to unneeded risks of treatment and treatment related side effects. Other patients forgo treatment and periodic testing for cancers that are judged low or immediate grade, which turn out to be aggressive. In addition, approximately one-half of approximately 1.3 million initial prostate cancer biopsies conducted in the US annually are negative, resulting in a high re-biopsy rate and use of expensive MRI imaging and/or other tests to improve pathological detection of prostate cancer. An estimated 80 percent of the 700,000 men who undergo second biopsies are subsequently diagnosed with prostate cancer.
The market potential for a precision prostate risk stratification test is estimated at $4 billion.
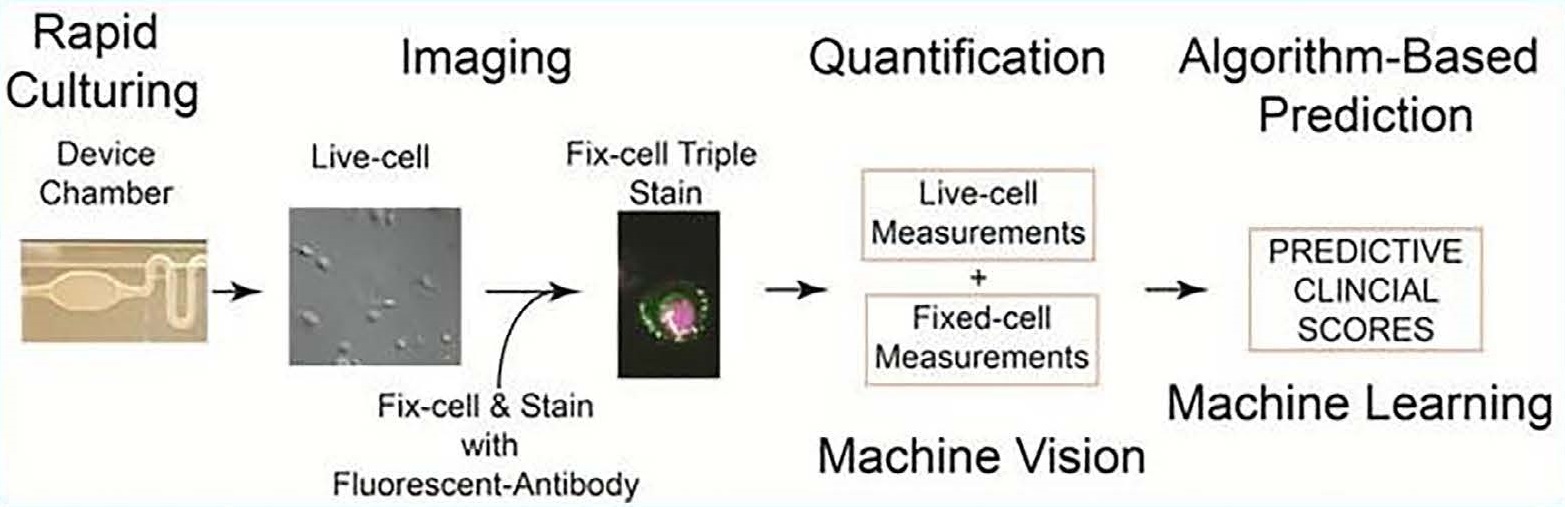
The live prostate cell phenotypic (LPCP) test entails the process of rapidly culturing primary prostate cells within 72 hours, imaging and quantification of both live and fixed cells, matching live and fixed cell measurements to be input into machine learning algorithms.
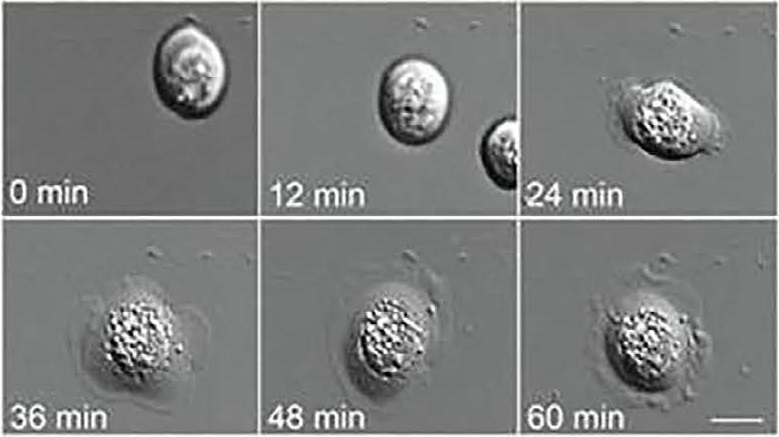
Live-cell biomarkers are measured via Differential Interference Contrast (DIC) microscopy that allows time-course imaging of primary prostate cells from tissue samples.
Cellanyx’s First-in-Class Prostate Test
Tumor cells act differently than normal cells. The Cellanyx test is the first to assess prostate cancer risk based on phenotype, the behavior of live tumor cells obtained from a patient biopsy. The test is designed augment Gleason testing and provide an actionable, quantitative score to measure likelihood that individual’s risk that a tumor is aggressive or not.
In a proof-of-concept study, published in the journal Urology, the test predicted specific post-surgical adverse pathology features – the gold standard of prostate cancer clinical diagnosis – with a high degree of sensitivity and specificity. The study was a multi-center, blinded, prospective trial that evaluated fresh prostate tissue samples taken from 251 men undergoing radical prostatectomy, of which 237 samples were successfully cultured and analyzed. The predictions of specific adverse pathology features based on the Cellanyx test were then compared to the actual post-surgical pathology reported findings following data un-blinding.
Based on the initial success with the proof-of-concept study, Cellanyx plans to conduct a prospective clinical study in prostate cancer patients at the time of needle biopsy to evaluate its ability to predict adverse pathology at the time of surgery. The study is designed to demonstrate the test’s clinical utility and will provide the basis for commercialization of the LDT.
Cellanyx’s LDT will be used in conjunction with the prostate pathology Gleason scoring test. The Cellanyx test, which delivers a result in three days, integrates with the workflows of both urologists and testing laboratories. The Company is exploring the potential for commercializing the test through the FDA 510(k) regulatory pathway.
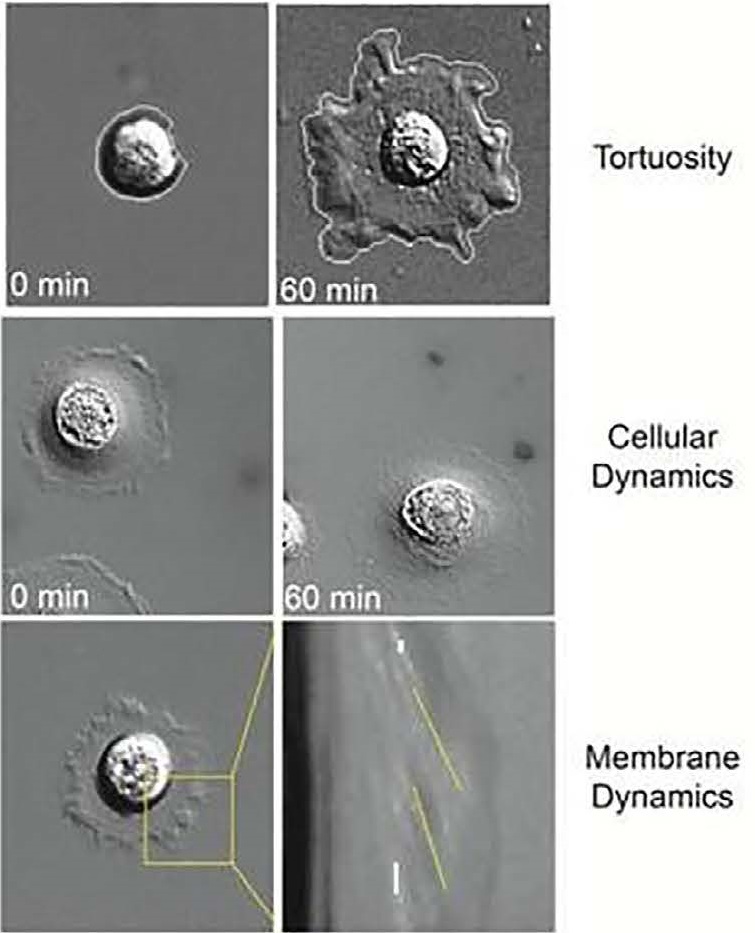
Examples of phenotypic biomarkers measured during live tumor cell analysis include tortuosity, cellular dynamics such as motility, and membrane dynamics that provide information about actin dynamics.
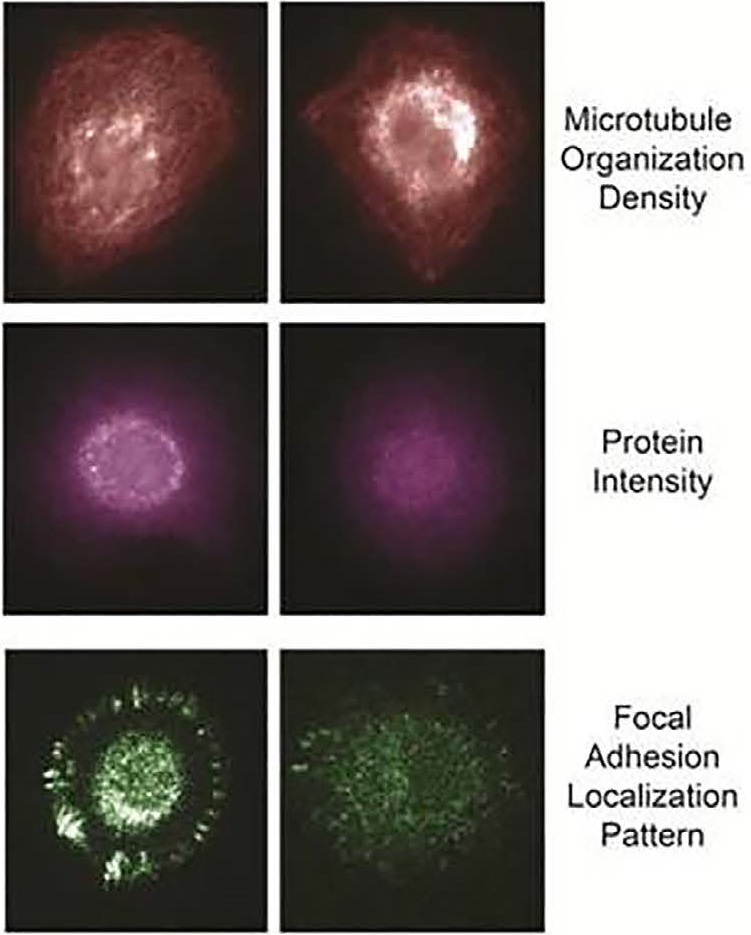
Example of fixed-biomarker measurements following live tumor cell analyses include microtubule organization, protein intensity and modification state, and focal adhesion localization pattern.
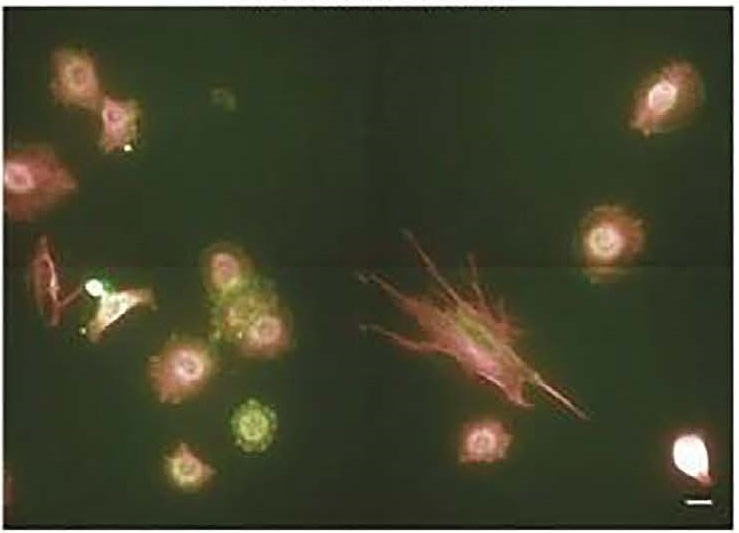
A representative field of view for label-based fixed-biomarker cell analysis.

